Dive deep into the world of drugs
WHO definition of drug:
A drug is any substance or product that is used or is intended to be used to modify or explore physiological systems or pathological states for the benefit of the recipient.
Drug is a chemical substance that is used for diagnosis, prevention & treatment of disease. The term “drugs” is often used to refer to addictive, abused, or illicit substances. However, this narrow and negative usage is a disservice to the original meaning of the term. “Drug” should be used to describe a substance with therapeutic or diagnostic purposes.
Nature of drugs:
Drugs are all made up of chemical substances, some having simple molecules while others having more complex ones. The majority of drugs are organic compounds, but there are also some that are purely inorganic, like lithium carbonate, ferrous sulfate, and magnesium hydroxide. Organic drugs can be either weakly acidic, like aspirin and penicillin, weakly basic, like morphine and chloroquine, or nonelectrolytes, like alcohol and diethyl-ether. Most drugs are commonly found in solid form, such as paracetamol, propranolol, furosemide, and ampicillin, but there are also liquids like ethanol, glyceryl trinitrate, propofol, and castor oil, as well as gaseous substances like nitrous oxide. The majority of drugs have a molecular weight ranging from 100-1000 D. Molecules that are smaller than 100 D usually lack specific features needed to bind selectively to target biomolecules. Conversely, molecules larger than 1000 D often struggle to pass through membranes to reach target sites in the body. Some drugs, such as lithium ion (7D), heparin (10-20 KD), gonadotropins (>30 KD), enzymes, proteins, and antibodies (>50 KD), vary greatly in size. Bulky molecule drugs typically require parenteral administration.
Sources of drugs:
-
Plants:
-
Alkaloids:
- Atropine (Atropa belladona)
- Morphine (Papaver somniferum)
-
Glycosides:
- Digoxin (Digitalis purpurea)
-
Oils:
-
Essential oil (Volatile oil):
- Leaves & Flower eg- clove oil, peppermint, eucalyptus
-
Fixed oil (nonvolatile):
- Seeds eg- groundnut, coconut, castor, olive oil
- Mineral oil: liquid paraffin
-
Essential oil (Volatile oil):
- Gum: excretory products (gum acacia)
- Resins: Tolu balsam (cough mix)
- Tannins: catechu
-
Alkaloids:
-
Animals:
- Hormones:
- Insulin (Pork-Procine), (Beef-Bovine)
- Vaccines: Polio, Anti-rabies
- Sera: ATS (Anti-tetanus Serum)
- Vitamins: Vitamin B12 from Liver extract
- Hormones:
- Microorganism: Antibiotics
- Chemicals: synthetic drugs
- Recombinant DNA Tech: Human Insulin, Calcitonin, Gonadotropins, erythropoietin etc.
- Minerals: Calcium salts, iron salts, iodine, magnesium/aluminum hydroxide
Drug nomenclature:
A drug generally has three categories of names:
1. Chemical name:
When a new chemical entity/substance is developed, a chemical name is given. It is the name given to the drug in accordance with rules of chemical nomenclature established by the International Union of Pure and Applied Chemistry (IUPAC). It is useful for chemists or technical personnel as it provides the precise arrangement of atoms and atomic groups in the molecule. Sometimes the name or information may be too complex for prescribing, so they assign a code name for ease and simplicity before an official name is decided upon.
2. Non-Proprietary name:
It is a short name given to a drug that is not subject to proprietary rights. The nonproprietary name should always be concise and meaningful. This is used in discussion and textbooks. It is also called as generic name. There are two classes of non-proprietary names:
- Approved Name
- Official Names
A. Approved name:
This name is given to drug by bodies like United Stats Adopted Name Council (USAN) and British Approved Name (BAN) soon after its introduction. This name sometime referred to as generic name however this term is used to designate a chemical or pharmacological class of drugs such as Sulphonamide, Penicillin.
B. Official names:
It is the name approved by the national pharmacopeia commission and included in the official book i.e. Pharmacopeia.
3. Proprietary name:
Name given by pharmaceutical company for commercial purposes. The name is assinged by the manufacturer(s) and is his property or trademark. One drug may have multiple proprietary names from different pharmaceutical companies or manufacturers. It is also known as trade or brand name.
Advantages:
- The consistency of pharmacokinetics or efficacy does not change with the same brand.
- A single brand name for a medicine with multiple ingredients.
- Bioavailability remains the same when a patient is maintained on a particular brand.
Disadvantages:
- Branded medicines are costlier.
- Multiple brands for the same medicine.
Drug Compendia:
Drug compendia are detailed guides that offer data on pharmaceutical products, containing details such as their ingredients, how they are made, their uses, recommended doses, potential interactions, and other important information. These resources are considered trustworthy sources of information on medications for healthcare professionals, regulatory bodies, scientists, and others engaged in the use, oversight, and study of drugs
Sources of drug information:
-
Official Compendia:
- a. Pharmacopoeia – Indian (IP), British (BP), United states (USP)
- b. Formulary – National Formulary of India (NFI), British National Formulary (BNF)
-
Non-Official Compendia:
- a. Physician’s Drug References (PDR) - USA
- b. Martindale Pharmacopoeia - Great Britain
-
Other Sources of Drug Information:
- a. Drug indices (CIMS, IDR, MIMS, Drug Index)
- b. Drug advertisement and Internet
Essential medicines:
WHO in 1977 published a list of drugs as “Model list of Essential drugs”. “List of drugs that satisfy the health care needs of majority of the population; they should, therefore be available at all times in adequate amount & in appropriate dosage form.” These drugs are selected based on their efficacy, safety, and cost-effectiveness, and they address the most prevalent health conditions and public health priorities. The current WHO list is revised in 2011 as 17th edition for adults with 23 FDC & 3rd edition for children. India produced its National Essential Drug List in 1996, presently it is revised in 2011 and 2015 with title “NLEM (National List of Essential Medicines)” which includes 376 medicines, of which 20 are FDCs.
Drug Dosage Forms:
Definition: It is the suitable form of a Drug that can be administrated to a patient.
Importance of Dosage forms:
- Dosage form provides a physical body to the drug.
- It demarcates the single dose of the drug.
- It protects the active ingredients of the drug.
- It makes the drug suitable for administration.
Classification of Dosage forms:
Dosage forms are classified based on their consistency. They are
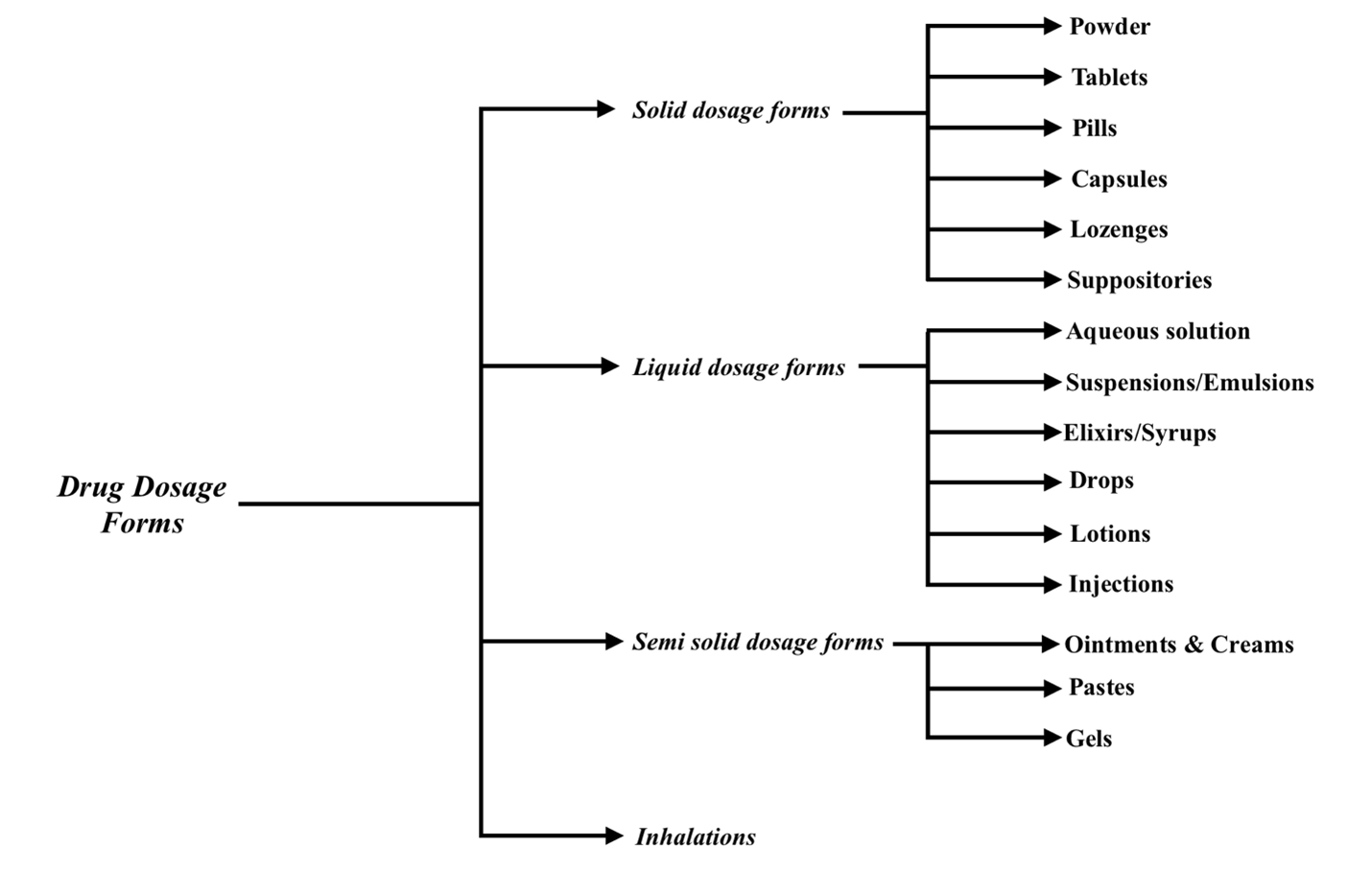
1. Solid Dosage Forms:
They can be further classified as follows:
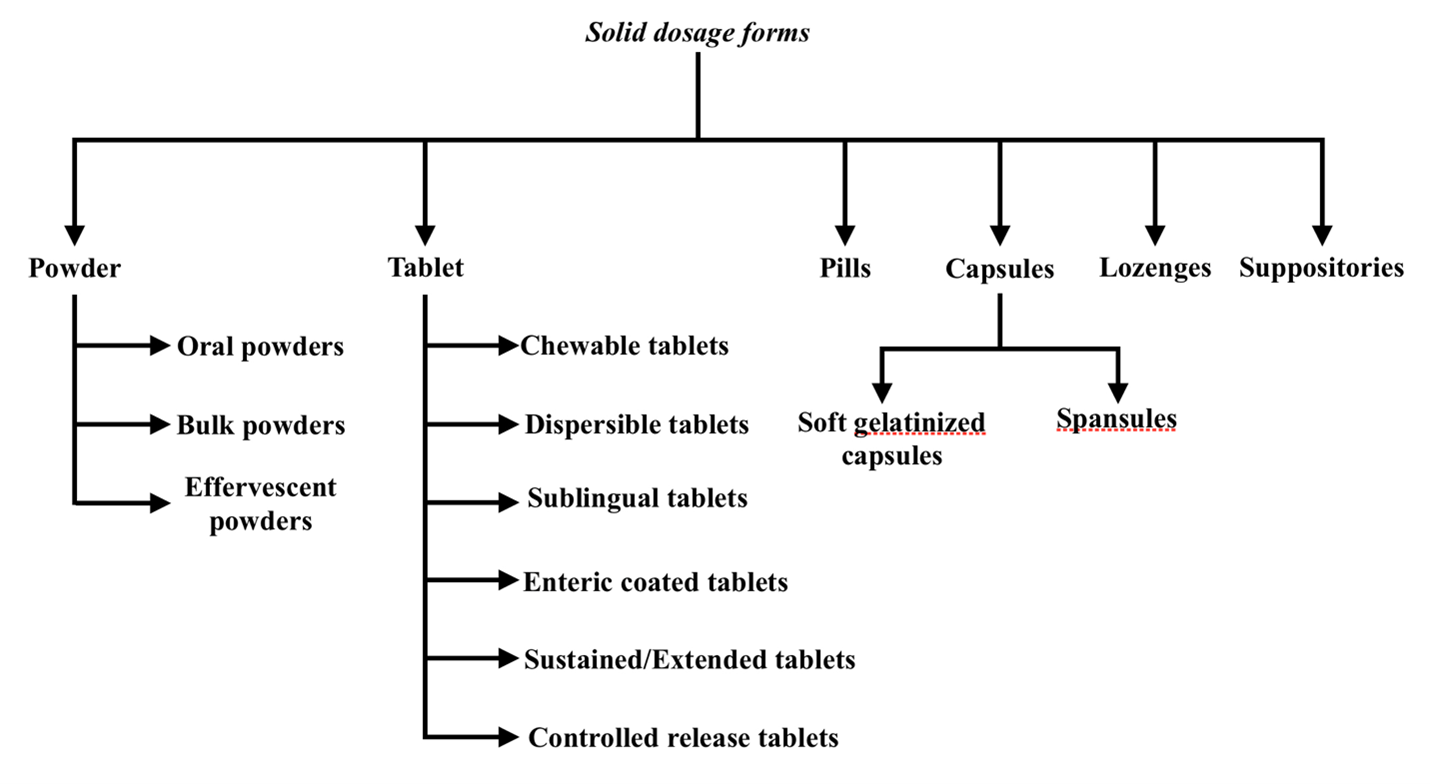
A. Powder form:
-
The drug is in dry and pulverized form.
-
Oral powders:
- Each dose of the drug is wrapped or packed in sachets separately.
- This form became inconvenient and unpopular, except when the quantity is in several grams.
Eg: ORS (Oral Rehydration Solution)
-
Bulk powders:
- Powders for tropical use stored in metallic or plastic containers.
Eg: Dusting powders
-
Effervescent powders:
- They contain granulated sodium bicarbonate and citric or tartaric acids leading to the production of CO2.
Eg: Eno
-
Oral powders:
B. Tablet form:
- Drug is in Granulated or powered form which is molded into desired shape.
- Active form of the drug mixed with inactive substances for to create a pharmaceutical formulation.
- They are molded into different shapes according to the suitable need of swallowing.
- They may or may not be coated with sugar.
-
Types:
-
Chewable tablets:
- Mostly used for children's.
- Ingredients must be pleasant.
Eg: Sorbitol and Mannitol
-
Dispersible tablets:
- Dissolved in water to form a solution for administration.
Eg: Aspirin is available as a dispersible tablet.
-
Sublingual tablets:
- Kept under the tongue for rapid absorption.
- The best example is Nitrostat used to relieve angina.
-
Enteric coated tablets:
- Coated with material that does not dissolve in an acidic medium.
- Used when disintegration needs to occur beyond the stomach, like in the duodenum.
- Commonly used materials for enteric coating include shellac, cellulose acetate phthalate, cellulose acetate butyrate, and some lipids.
Eg: Aspirin, Pantoprazole, Omeprazole
-
Sustained/Extended tablets:
- These are coated drugs that dissolve at a specific rate.
- Benefit: Active ingredient is available for a longer period, extending the drug's action time (e.g., from 4 hours to 12 hours).
Eg: Alprazolam, Estradiol
-
Controlled release tablets:
- A semi-permeable membrane is present to control the release of the drug.
Eg: Plendil ER (Felodipine), Agon SR (Felodipine), Kapanol (Morphine sulphate)
-
Chewable tablets:
C. Pills:
- It is one of the oldest dosage form of drugs.
- These are mixed with honey or syrup and make a sticky mass. Then it was rolled and swallowed.
Eg: Phenobarbital and oxycodone
D. Capsules:
- Water-soluble cylindrical gelatinized containers filled with medications
-
Soft gelatinized capsules:
- They contain liquid medications which can be dissolved rapidly.
Eg: Cyclosporine, Declomycin, Vitamin A and E.
-
Spansules:
- Drug is packed in granular form with different coatings so that they can be dissolved at different intervals.
Eg: Dexedrine spansule capsules
E. Lozenges:
- Drugs are in tablet-like bodies with various shapes.
- They contain the drug along with suitable gum and flavoring agents.
Eg: Vicks and strepsils
F. Suppositories:
- Conical bullet-shaped dosage forms inserted into the anal canal, especially used in children.
- The drug is present in a moldable firm base which is melted to the body temperature and then the drug is released.
- A similar mechanism is used in:
- Pessaries - Oval shaped forms for vaginal insertion.
- Bougies - Pencil shaped cones inserted into the urethra (male and female).
2. Liquid Dosage Forms:
These can be further classified as follows:
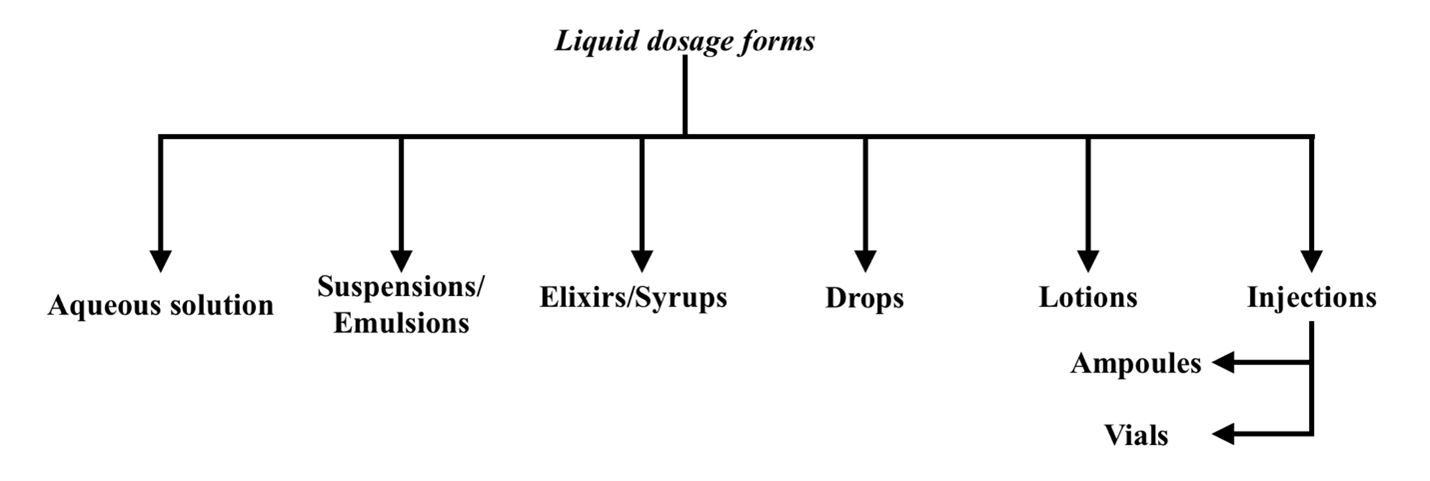
A) Aqueous Solutions:
- Drug is dissolved in water and meant for oral, tropical, and parenteral administration.
- Oral drug solutions contain flavoring agents and preservatives.
- Mouthwashes are well-known examples.
B) Suspensions/Emulsions:
- Suspension is solid in liquid and Emulsion is liquid in liquid.
- A dispersion of insoluble drugs in water with the help of a suspending agent.
- Both the suspension and emulsions tend to settle down on keeping, so they should be shaken well before use.
- Oral antacids are available as suspensions.
C) Elixirs/syrups:
- Elixirs are hydro-alcoholic solutions of drugs.
- Elixirs are slightly different from syrups in having less viscous consistency and no sucrose at all.
- Elixirs are more stable than syrups because of having alcohol with water as the main solvent, whereas syrups have sugars and water as the main solvent.
- Dexamethasone, Fluphenazine are commonly used medicated elixirs.
D) Drops:
- These are relatively more concentrated than the previous ones.
- These are meant for oral administration or external applications like eyes, nose, ear canal, etc.
- Oral drops are more preferable for infants.
- Eye drops need to be sterilized at the time of preparation and use.
E) Lotions:
- These are available in solutions, suspension, or emulsion forms which are meant only for external use.
- These are better preferred for hairy skin than creams and ointments.
F) Injections:
- These can be given subcutaneously, Intramuscular (IM), Intra Venous (IV).
- These are sterile solutions or suspensions in aqueous or oily medications that are given according to their requirements.
- I.V injections are only given as aqueous solutions not as suspensions because suspensions and oil medications that are given in I.V can cause embolism.
- Injections are available in both Ampoules and Vials.
a. Ampoules:
- These are available as sealed glass tubes which are broken just before use.
- Usually available as a single dose.
b. Vials:
- These are airtight rubber-capped small bottles available as both single and multi-dosage forms.
- Unstable aqueous drugs are supplied as dry powder which is used along with sterile solution before use.
- Drugs like insulin are available as prefilled syringes.
- Large volumes of I.V infusions are supplied in glass or polypropylene bottles.
3. Semi solid dosage forms:
These can be further classified as follows:
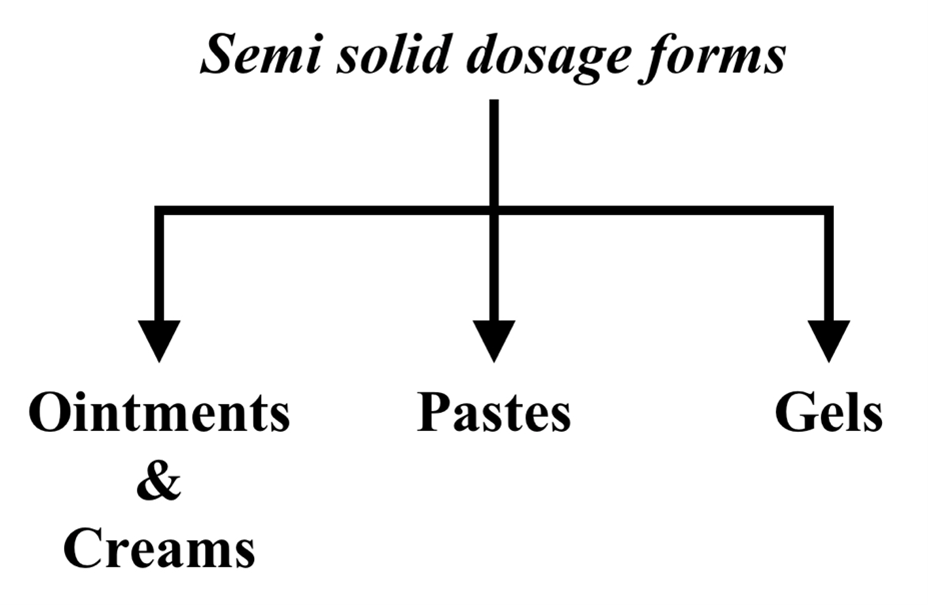
Ointments & Creams:
- These are greasy semi-solid drug forms meant for external application to the skin, eyes, nasal mucosa, ear, and anal canal.
- Note: These are not suitable for applying on oozing surfaces because of their occlusive nature and do not allow water to evaporate.
- They are different from creams in having an oily base like paraffin, beeswax, etc. Creams have water-in-oil emulsion as a base.
- Creams are better absorbed medications to the skin and are cosmetically more acceptable than ointments.
Eg: Hydrocortisone cream, Neosporin ointment, and Eucerin Aquaphor healing ointment
B) Pastes:
- These are non-greasy forms of thick consistency containing hydrophilic adhesion powders (such as chalk, carboxy methyl cellulose etc..) which swells by absorbing water.
- These can be applied on inflamed, excoriated skin and on oozing surfaces (contrast to ointments) and mucous membranes.
Eg: Toothpastes
C) Gels:
- These are medications of viscous colloidal solutions of gelatin or similar products.
- These drugs are generally available in collapsible tubes, and they are meant for external applications to the skin and mucous membranes.
- They provide a longer duration of contact with non-greasy and washable water.
- These drug forms are more suitable for hairy skin and commonly applied to oral ulcers.
Eg: Hyaluronic acid and Hand sanitizer
4) Inhalations:
- These are drug forms that are gases or volatile liquids that can be administered by inhalation, carried into the air or oxygen with the help of a mouthpiece, face mask, hood, or endotracheal tube.
- Nonvolatile liquids and fine solids can be aerosolized using inhalers and nebulizers.
- Pressurized metered dose inhalers (PMDIs) are handheld devices that use a propellant (substances that give a forward thrust), mostly hydrofluoroalkanes (HFA), which deliver a specialized dose of the drug in aerosol form per actuation.
- The efficacy of the aerosolized drug depends on the particle size: 1-5 µm diameter particles deposit on the bronchioles and effectively deliver the drug.
Eg: Albuterol inhaler
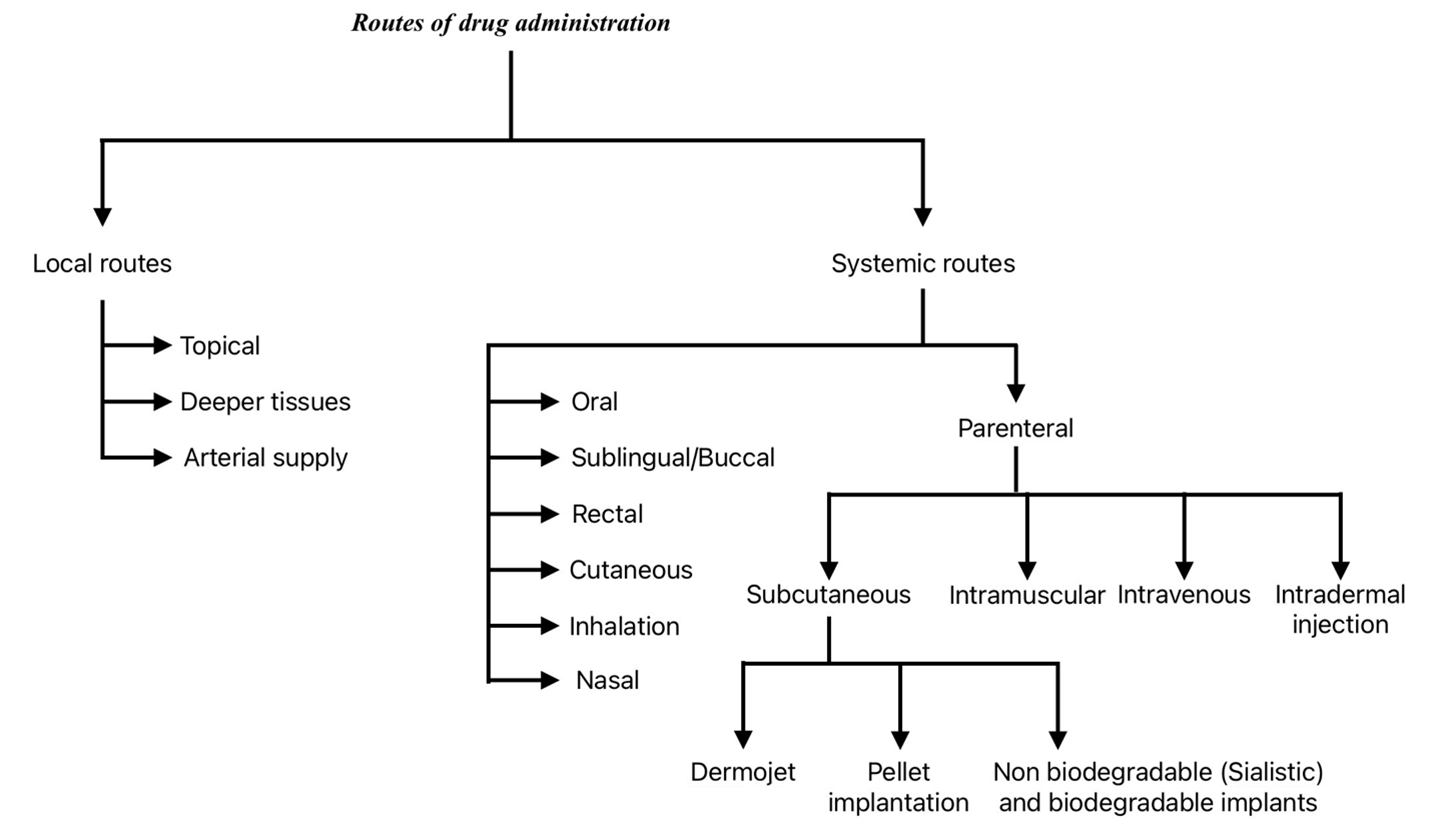
Routes of drug administration:
A route of administration is a way that a drug can enter the body. There are many drug routes of administration. The choice of appropriate route in a given situation depends both on drug as well as patient related factors.
Classification of drug administration: